Dr. John Flake's Research Group
Publications
Google Scholar
Research
Electrocatalysis
Electrochemical reduction of CO2:
CO2 concentrations in the Earth’s atmosphere have been on a dramatic rise over the past century, which directly results in an increase in temperature anomalies and deleterious climate change effects (global warming). As a result, developing sustainable technologies that can transfer CO2 into value added chemicals provides a promising pathway to reduce the rising levels of CO2 emissions. Our group aims at providing electrochemical transformations of CO2 into value added chemicals (such as HCOOH, CH3OH). Electrochemical reduction of CO2 provides an opportunity to store renewable energy as fuels with much greater energy densities than batteries. Product selectivity of the reduction reaction is known to be a function of the electrolyte and electrode. Our aim is to improve the product selectivity (Faradaic efficiency), energetic efficiency and conversion rate (current density) through the development of suitable electrocatalysts, and modification of the electrode surface.
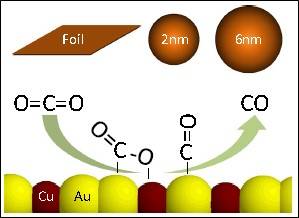
Electrochemical oxidation of CH4:
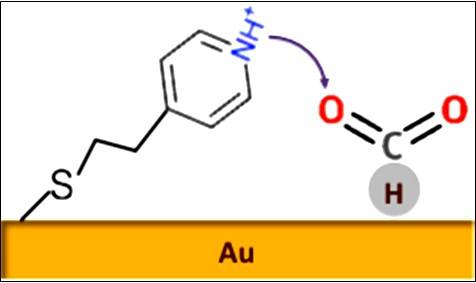
Methane is abundant in nature including approximately ~6,000,000 Tg located undersea or underground and 5000 Tg (~1%) in the atmosphere. In the last 200 years, atmospheric methane concentrations have doubled from 0.8 ppm to 1.6 ppm. This is troubling, particularly because methane has a global warming potential 23 times greater than CO2. Unfortunately, conventional methane to hydrocarbon conversion routes (e.g. Fischer-Tropsch, acetylene) are relatively inefficient and have not widely displaced hydrocarbons sourced from crude refining or ethane cracking. Our group aims at providing efficient electrochemical methods to selectively convert CH4 to specific hydrocarbons to higher hydrocarbons (C2+) without oxygenated or halogenated intermediates at high yields and efficiencies through modulating potential, electrolyte and electrode.
Batteries
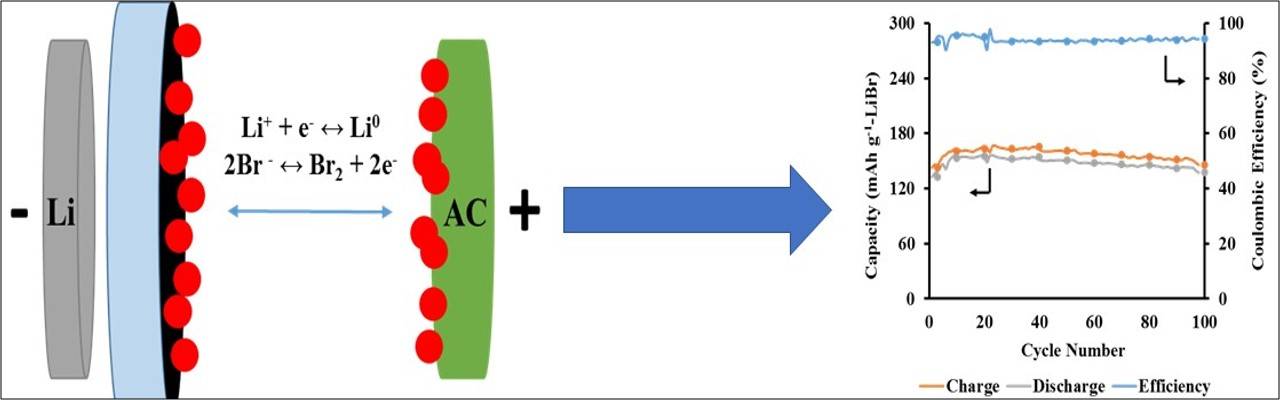
Lithium-air and lithium-sulfur batteries have been proposed as potential higher energy capacity alternatives; however, various issues plague these battery types, ultimately affecting the energy and performance. Lithium-air batteries suffer from poor cyclability (~ 60-70% efficiency) and materials issues stemming from moisture and poor separation of environmental contaminants, while capacity fading (> 10% over 100 cycles) and loss of active material remain huge challenges for lithium-sulfur batteries due to polysulfide dissolution. Our group aims at developing a new generation of lithium-based batteries using a lithium-bromide chemistry which have shown promising results as a rechargeable alternative to lithium-ion batteries, including energy densities and efficiencies.
Semiconductors
Flip chip joint quality and reliability are strong functions of several parameters including the under bump metallization (UBM), type of solder, and reflow process and flux. In the UBM stack, Cu pads may be coated with adhesion layers, diffusion barriers and capped with a metal such as Ni for flip-chip soldering; other device manufactures simply use the Cu pads as the last metal. Before reflow, the surfaces to be joined are treated with an acid flux to remove oxides and promote wetting. While the mechanical properties, materials and processes associated with flip-chip packaging are relatively well understood, there are few studies aimed at understanding flux reactions between acids and UBM surfaces or solders. Our group aims at studying metal oxides (such as CuOx, SnOx, and NiO) removal effectiveness of nonaqueous acid using electrochemical methods.
![]()
Funding Support
National Science Foundation

Intel

Lab Photos





