Research Areas
Experimental investigation of cardiovascular function, diseases, and therapies
In vitro, in vivo, and ex vivo assessments of cellular and tissue properties continue to play a crucial role in enhancing our understanding of (patho)physiology since the confounding genetic, mechanobiological, and immunological contributions to tissue maintenance, disease, and adaptation are often impossible to untangle using clinical data alone. Using animal models, we study myocardial, valvular, and vascular physiology under a variety of non-pathological (e.g., maturation, pregnancy) and pathological (e.g., genetic knockout, cardiovascular injury) conditions, with a current focus on congenital defects and other diseases that affect multiple tissue types. Integrating quantitative multimodal data, including subject-specific anatomy/geometry, mechanical properties, extracellular matrix composition/architecture, and gene expression, we seek to develop (1) logic-based network models to identify key underlying signaling pathways in relevant cell populations, (2) mathematical models to describe time-evolving remodeling of relevant tissues, and (3) computational models to capture organ-level biomechanical interactions.
Representative past projects
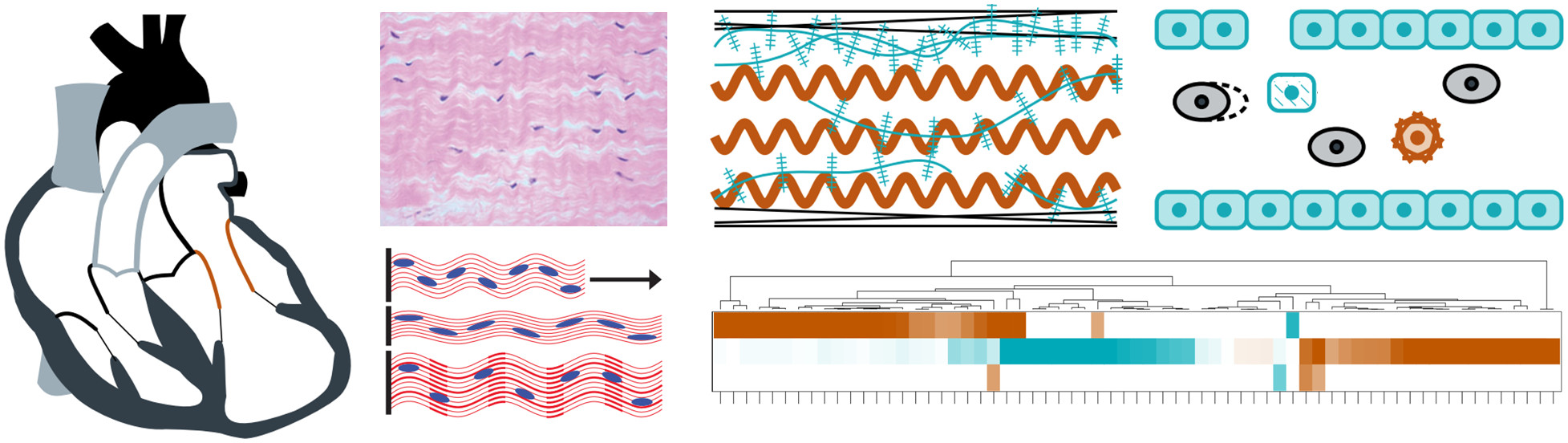
We have investigated the effects of altered loading conditions on the mitral valve and the role of interstitial cells in mediating extracellular matrix remodeling. Throughout pregnancy, which represents a non-pathological cardiac volume overload, we found that the maternal mitral valve undergoes an initial period of permanent passive leaflet distention followed by a longer-term adaptive restoration of tissue-level behavior and cellular geometry.1 In a separate study on mitral valve remodeling after myocardial infarction, we found similarly that there was significant plastic deformation of the leaflet tissue, accompanied by altered composition and gene expression.2 Both of these studies suggest that mitral valve interstitial cells respond to the time-averaged mechanical loading experienced by the leaflet, and that tissue-level remodeling is achieved by cell-mediated turnover of extracellular matrix constituents. These findings have implications for understanding and predicting mitral valve growth and remodeling in various clinical scenarios.
Publications:
- Rego BV, Wells SM, Lee CH, Sacks MS. "Mitral valve leaflet remodelling during pregnancy: insights into cell-mediated recovery of tissue homeostasis." Journal of the Royal Society Interface. 2016 Dec 31;13(125):20160709.
- Howsmon DP,* Rego BV,* Castillero E, Ayoub S, Khalighi AH, Gorman RC, Gorman JH, Ferrari G, Sacks MS. "Mitral valve leaflet response to ischaemic mitral regurgitation: from gene expression to tissue remodelling." Journal of the Royal Society Interface. 2020 May 06;17(166):20200098. *These authors contributed equally.
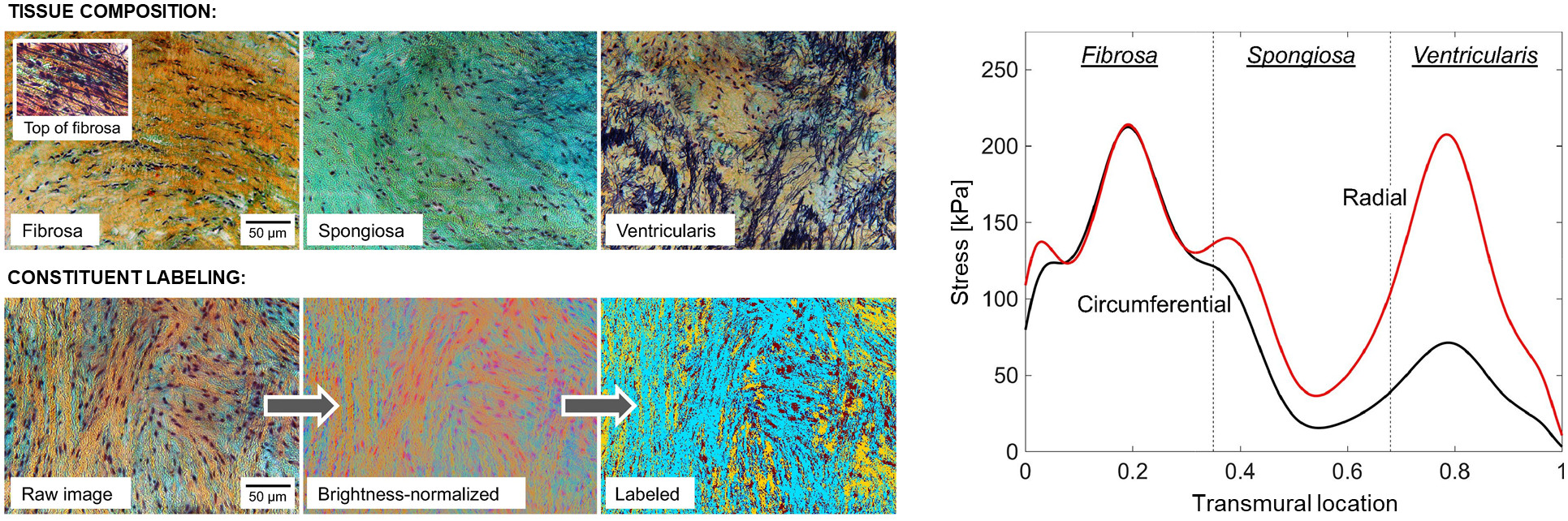
Heterogeneities in structure and stress within heart valve leaflets are of significant concern to their functional physiology, as they affect how the tissue constituents remodel in response to both pathological and non-pathological alterations in cardiac function. Quantifying local variations in matrix structure and stress is thus necessary to understand homeostatic valve maintenance as well as to develop predictive models of disease progression and post-surgical outcomes. We developed a new structural constitutive model for the aortic valve leaflet that treats the leaflet as a functionally graded material whose properties vary continuously over the thickness. The model predicted large stress variations both between and within the leaflet’s tissue layers, suggesting that the continually varying structure of the leaflet has an important purpose with regard to valve function and tissue homeostasis.
Publication:
Rego BV, Sacks MS. "A functionally graded material model for the transmural stress distribution of the aortic valve leaflet." Journal of Biomechanics. 2017 Mar 21;54:88-95.
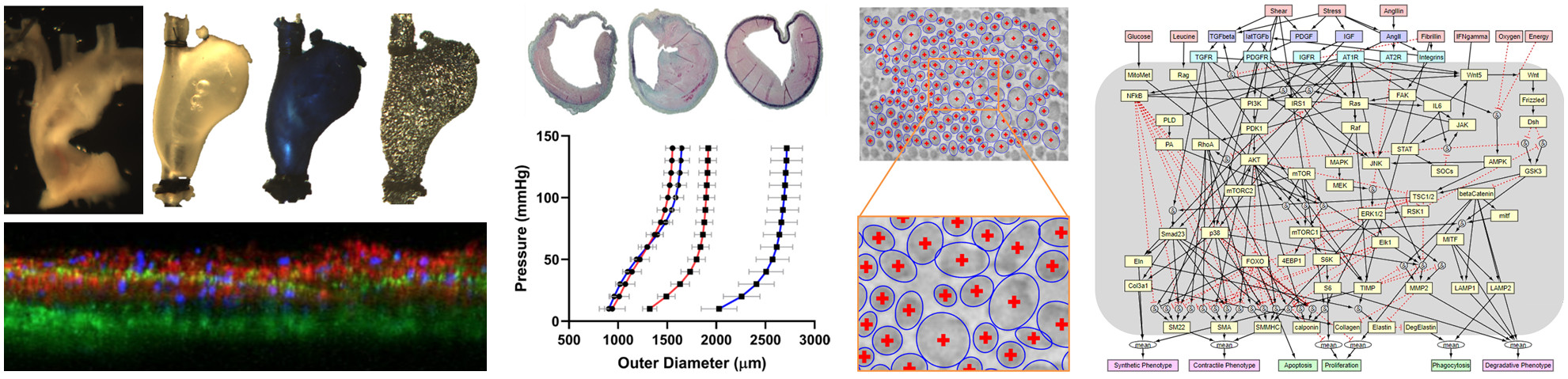
Aortopathies (aneurysms, dissections, and rupture) are increasingly responsible for significant morbidity and mortality, but the underlying mechanobiological mechanisms that govern disease progression remain poorly understood. We have performed detailed investigations of aortic microstructure and mechanical properties in the context of abdominal aortic aneurysms1 and Marfan syndrome.2 In both studies, we combined ex vivo biaxial mechanical testing with microscopic and histological examinations to quantify regional heterogeneities in deformation, stress, and stiffness, which provided new insights into how both pathologies are characterized by dysfunction of collagen and especially elastin within the aortic wall. We have also developed a network model of vascular smooth muscle cell signaling and used it to capture the effects of a Tsc1 knockout, which has been implicated in thoracic aortopathies.3
Publications:
- Weiss D,* Latorre M,* Rego BV, Cavinato C, Tanski BJ, Berman AG, Goergen CJ, Humphrey JD. "Biomechanical consequences of compromised elastic fiber integrity and matrix cross-linking on abdominal aortic aneurysmal enlargement." Acta Biomaterialia. 2021 Jul 29;134:422-434. *These authors contributed equally.
- Weiss D, Rego BV, Cavinato C, Li DS, Kawamura Y, Emuna N, Humphrey JD. "Effects of age, sex, and extracellular matrix integrity on aortic dilatation and rupture in a mouse model of Marfan syndrome." Arteriosclerosis, Thrombosis, and Vascular Biology. 2023 Sep 01;43(9):e358–e372.
- Estrada AC, Irons L, Rego BV, Li G, Tellides G, Humphrey JD. "Roles of mTOR in thoracic aortopathy understood by complex intracellular signaling interactions." PLoS Computational Biology. 2021 Dec 13;17(12):e1009683.
Image-based computational modeling for prognosis and treatment planning
We have previously developed image-based 3D geometric reconstruction, local mechanical characterization, and finite element simulation pipelines to build high-fidelity computational models of heart valves and blood vessels on a subject-specific basis. We seek to extend and combine these workflows to model heart valves and their atrioventricular or arterial neighbors under a unified framework that will incorporate cell- and tissue-level adaptation models informed by our experimental studies. These models will predict disease progression and therapeutic outcomes on a subject-specific basis, and we will validate our predictions against animal and human data (acquired in partnership with clinical, physician-scientist, and/or veterinary collaborators). Over the longer term, we will use this framework to design, optimize, and personalize novel therapies.
Representative past projects
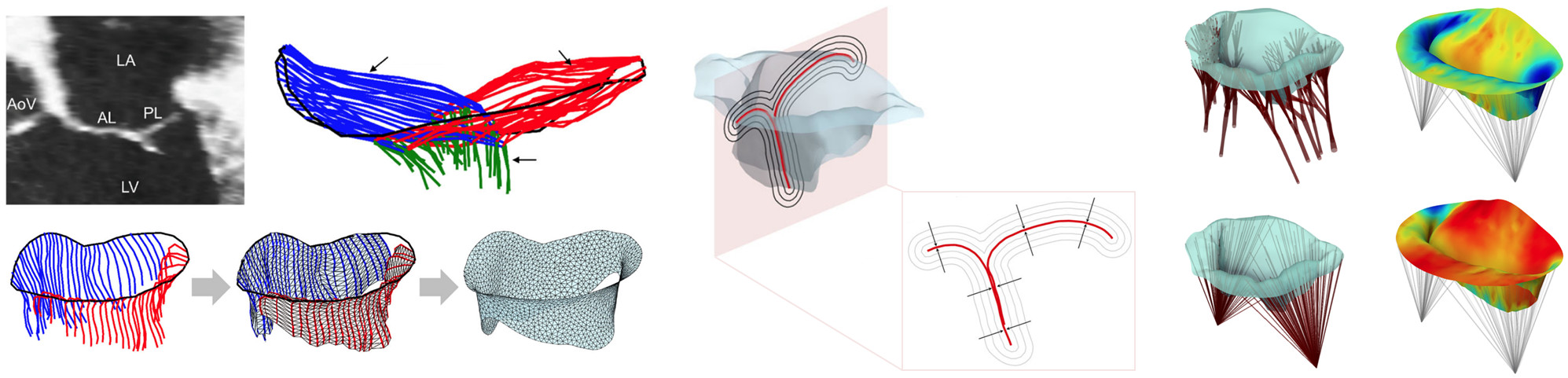
Assessment of mitral valve function is important in many diagnostic, prognostic, and surgical planning applications. Yet, there are no clinically available noninvasive methods for determination of leaflet deformations, which are a critical metric of valvular function, or for simulation of mitral valve function under a variety of potential therapeutic approaches. We have developed a completely noninvasive computational method to estimate local maps of leaflet deformations from clinical-quality 3D echocardiographic images.1 We have also developed a method to produce "functionally equivalent" models of the mitral valve chordae tendineae that can be built from the image-based leaflet geometry alone.2 Combining both approaches, we are now able to construct patient-specific computational models of the complete mitral valve apparatus, which can be used directly for prediction (rather than just evaluation) of mitral valve function under various disease and treatment scenarios. We have since applied our approach to quantify changes in mitral valve deformation patterns following myocardial infarction3 and to develop a predictive model of post-infarction mitral valve leaflet plasticity.4
Publications:
- Rego BV, Khalighi AH, Drach A, Lai EK, Pouch AM, Gorman RC, Gorman JH, Sacks MS. "A noninvasive method for the determination of in vivo mitral valve leaflet strains." International Journal for Numerical Methods in Biomedical Engineering. 2018 Dec 04;34(12):e3142.
- Khalighi AH, Rego BV, Drach A, Gorman RC, Gorman JH, Sacks MS. "Development of a functionally equivalent model of the mitral valve chordae tendineae through topology optimization." Annals of Biomedical Engineering. 2019 Jan 15;47(1):60-74.
- Rego BV, Khalighi AH, Lai EK, Gorman RC, Gorman JH, Sacks MS. "In vivo assessment of mitral valve leaflet remodelling following myocardial infarction." Scientific Reports. 2022 Oct 26;12:18012.
- Rego BV, Khalighi AH, Gorman JH, Gorman RC, Sacks MS. "Simulation of mitral valve plasticity in response to myocardial infarction." Annals of Biomedical Engineering. 2023 Jan 01;51(1):71-87.
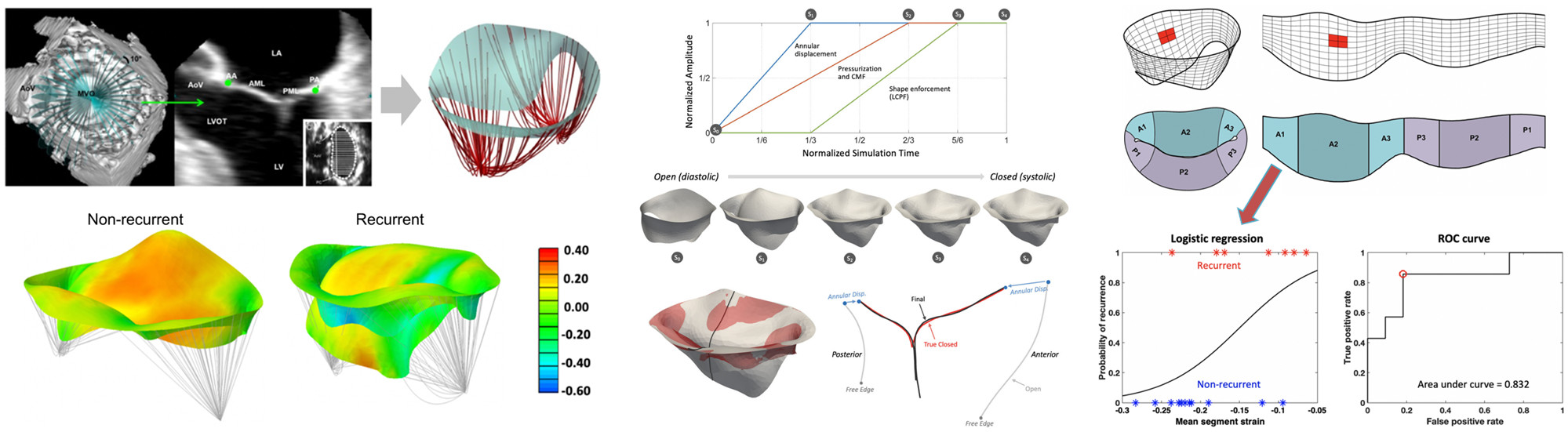
Ischemic mitral regurgitation is a prevalent cardiac disease associated with substantial morbidity and mortality. Contemporary surgical treatments continue to have limited long-term success, in part due to the complex and multi-factorial nature of the pathology. We have applied our noninvasive deformation estimation method to quantify peak systolic leaflet strains in human mitral valves from in vivo 3D echocardiographic images acquired immediately prior to and post-annuloplasty repair. We found statistically significant differences in pre-surgical mitral valve size, shape, and deformation patterns between patients with and without regurgitation recurrence six months after surgery. Our results suggest greater disease progression in the recurrent group and underscore the highly patient-specific nature of ischemic mitral regurgitation. Importantly, the ability to identify such factors pre-surgically could be used to guide optimal treatment methods to reduce post-surgical regurgitation recurrence.
Publication:
Narang H, Rego BV, Khalighi AH, Aly A, Pouch AM, Gorman RC, Gorman JH, Sacks MS. "Pre-surgical prediction of ischemic mitral regurgitation recurrence using in vivo mitral valve leaflet strains." Annals of Biomedical Engineering. 2021 Apr 09;49(12):3711-3723.
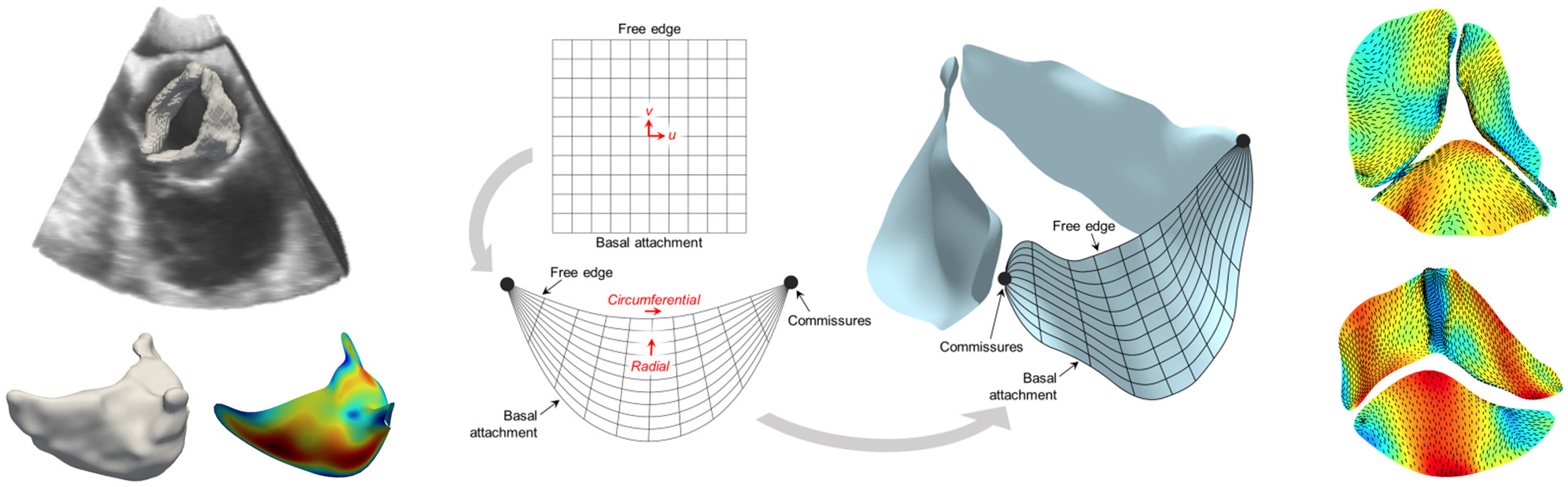
Bicuspid aortic valve disease is the most common cardiac congenital defect in humans and can lead to premature and severe aortic stenosis or insufficiency. However, patient-specific risk assessment is hampered by the substantial degree of anatomic and functional variations that remain largely unknown. We utilized our noninvasive computational pipeline to infer local heart valve leaflet deformation information using 3D echocardiographic images from patients with normal and bicuspid aortic valves. The resulting deformation analysis resulted in, for the first time, quantified differences between the in vivo functional deformations of the normal and bicuspid aortic valve leaflets. We were able to identify and quantify differences in stretch patterns between leaflet types, and found in particular that stretches experienced by bicuspid aortic valve leaflets during closure differ from those of normal aortic valve leaflets in terms of both heterogeneity as well as overall magnitude. This study is an essential step toward patient-specific assessment of bicuspid aortic valve disease based on correlating leaflet deformation and aortic stenosis/insufficiency progression.
Publication:
Rego BV, Pouch AM, Gorman JH, Gorman RC, Sacks MS. "Patient-specific quantification of normal and bicuspid aortic valve leaflet deformations from clinically derived images." Annals of Biomedical Engineering. 2022 Jan 07;50(1):1-15.
Machine learning and data science for real-time biomechanical simulation
Recent advances in machine learning have enabled fast surrogates for high-fidelity models. While not a replacement for mechanistic models (such as those developed in our other research areas), these surrogates overcome computational hurdles that otherwise make such modeling intractable within clinically relevant time frames. Particular frameworks, such as physics-informed neural networks (PINNs) and deep operator networks (DeepONets), have proven especially valuable in biomedicine since they enable model calibration with less data. For cardiovascular applications, such models have shown promise in risk stratification and prediction of disease progression. We seek to combine machine learning platforms with mechanistic and phenomenological models to predict long-term progression of cardiovascular diseases and therapeutic outcomes. Toward this end, we will also develop statistical approaches to facilitate multi-resolution modeling, model selection, credibility analyses, uncertainty quantification, and probabilistic prediction.
Representative past projects
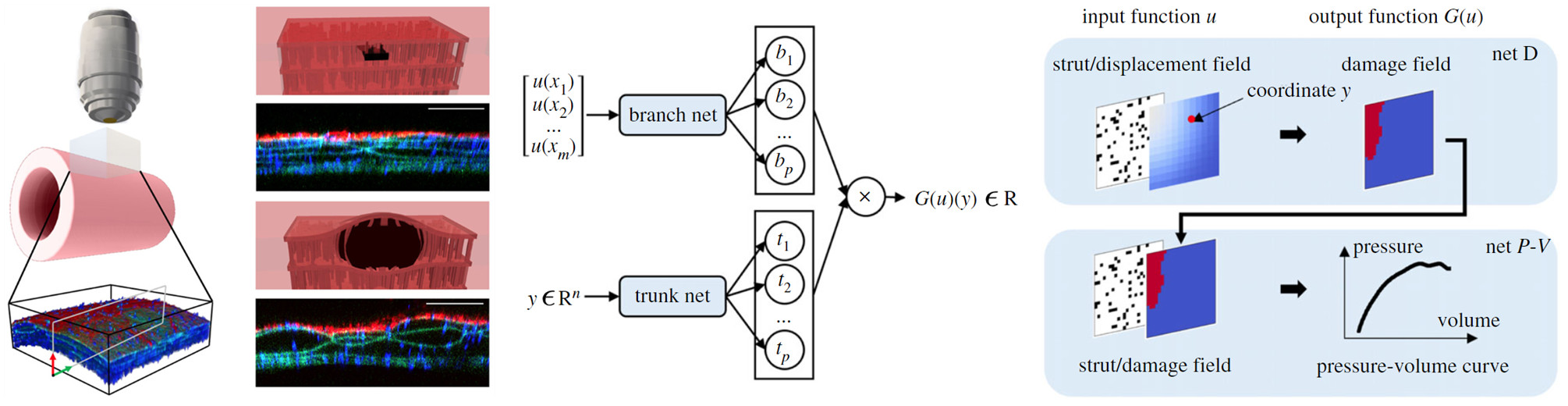
The differential propensity of dissection along the aorta can be affected by spatial distributions of structurally significant interlamellar struts that connect adjacent elastic lamellae within the medial layer of the wall. We have developed a data-driven surrogate model of the delamination process for differential strut distributions using a DeepONet. This surrogate model was trained to predict the pressure–volume curve of the injected fluid and the damage progression within the wall given a spatial distribution of struts, with in silico data generated using a phase-field finite-element model. The results show that the DeepONet can provide accurate predictions for diverse strut distributions, indicating that this composite branch–trunk neural network can effectively extract the underlying functional relationship between distinctive microstructures and their mechanical properties. More broadly, DeepONets can facilitate surrogate model-based analyses to quantify biological variability, improve inverse design, and predict mechanical properties based on multi-modality experimental data.
Publication:
Yin M, Ban E, Rego BV, Zhang E, Cavinato C, Humphrey JD, Karniadakis GE. "Simulating progressive intramural damage leading to aortic dissection using DeepONet: an operator–regression neural network." Journal of the Royal Society Interface. 2022 Feb 09;19(187):20210670.
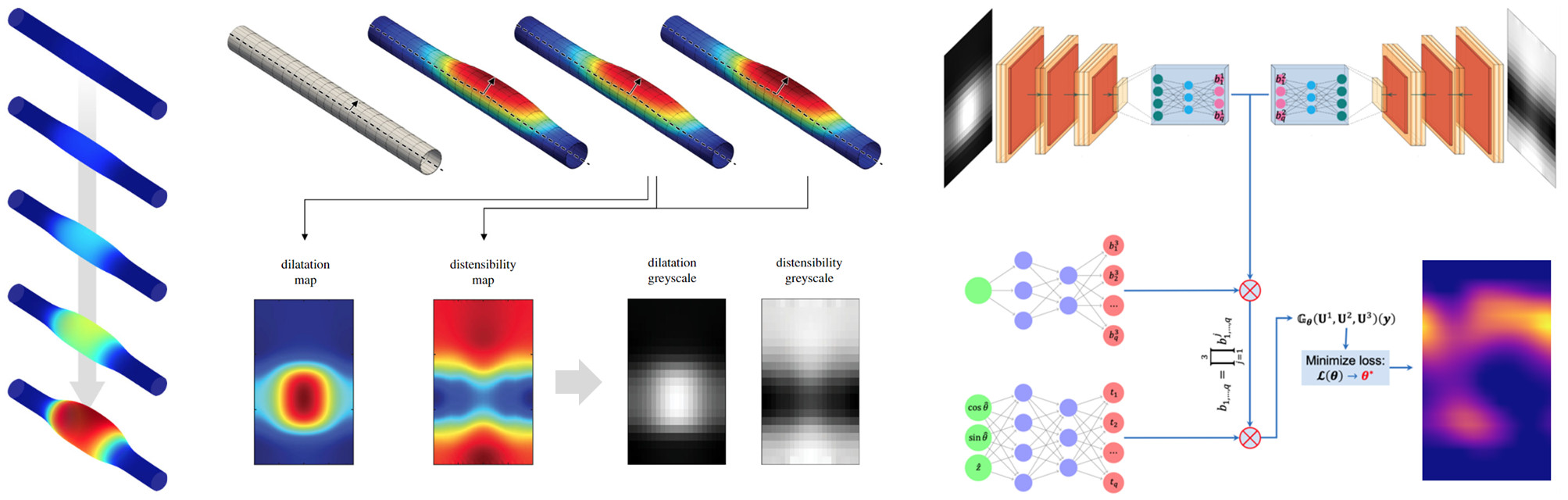
In vivo assessments of aneurysm progression are largely limited to measurements of aneurysm size and growth rate. There is promise, however, that computational modeling of the evolving biomechanics of the aorta could predict future geometry and properties from initiating mechanobiological insults. We have developed an integrated framework to train a DeepONet-based surrogate model to identify mechanobiological factors that contribute to aneurysm formation and enlargement. For training, we employed a constrained mixture model of aortic growth and remodeling to generate maps of local aortic dilatation and distensibility via finite element simulations. We evaluated the performance of the surrogate model for insult distributions varying from fusiform (analytically defined) to complex (randomly generated) and showed that this continuous learning approach can predict the vessel-specific insult profile associated with any given dilatation and distensibility map with high accuracy. Our findings demonstrate the feasibility of applying DeepONets to support transfer learning of patient-specific inputs to predict aneurysmal progression.
Publication:
Goswami S,* Li DS,* Rego BV, Latorre M, Humphrey JD, Karniadakis GE. "Neural operator learning of heterogeneous mechanobiological insults contributing to aortic aneurysms." Journal of the Royal Society Interface. 2022 Aug 31;19(193):20220410. *These authors contributed equally.
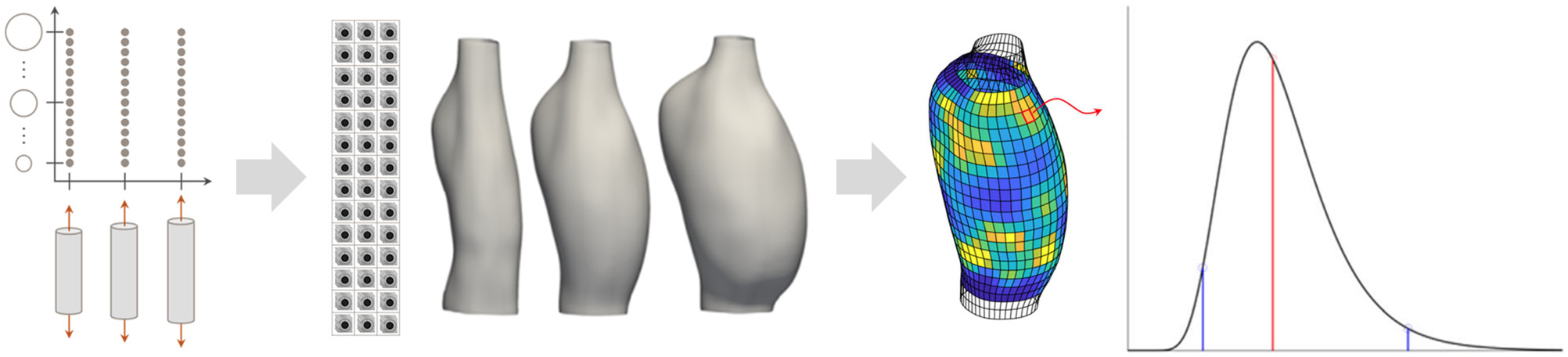
Quantitative estimation of local mechanical properties remains critically important in the ongoing effort to elucidate how blood vessels establish, maintain, or lose mechanical homeostasis. Recent advances based on panoramic digital image correlation have made high-fidelity 3D reconstructions of small-animal (e.g., murine) vessels possible when imaged in a variety of quasi-statically loaded configurations. However, the inability to quantify uncertainties associated with local point estimates of mechanical properties has compromised our ability to draw subject-specific conclusions from such data. We have addressed this limitation by integrating a novel uncertainty quantification and propagation pipeline within an inverse modeling approach, relying on empirical and analytic Bayesian techniques. Our extended workflow not only allows parameter uncertainties to be systematically reported, but also facilitates both subject-specific and group-level statistical analyses of the mechanics of the vessel wall.
Publication:
Rego BV, Weiss D, Bersi MR, Humphrey JD. "Uncertainty quantification in subject-specific estimation of local vessel mechanical properties." International Journal for Numerical Methods in Biomedical Engineering. 2021 Oct 04;37(12):e3535.